Investigating Immune Responses in Early-Onset Alzheimer’s Disease
By Anvi Murarka
Changes in immune signatures are becoming a key focus in neurodegenerative diseases, yet there is limited understanding of these immune responses in early-onset Alzheimer’s Disease (EOAD). EOAD affects individuals prior to the age of 65, and usually presents with episodic memory impairment and subsequent cognitive decline.
This study aimed to gain a better understanding of the disease risk by conducting unbiased single cell RNA sequencing (scRNA-Seq) analysis of peripheral blood mononuclear cells (PBMCs) in EOAD cases and healthy controls.
All the participants were recruited from the UCSF Memory and Aging Center and took part in a multi step screening process for neurodegenerative disease. They were diagnosed according to consensus criteria for amnestic Alzheimer’s or frontal Alzheimer’s Disease. All of the control individuals had a normal neurologic exam. PBMCs were obtained from study participants, analyzed by single cell RNA sequencing, and bioinformatic tools were used for cell clustering. Statistical differences in PBMCs abundances were assessed by linear modeling, controlling for age and sex. To uncover their distinct characteristics, we focused on early onset Alzheimer’s Disease (EOAD). This study included patients with sporadic early onset Alzheimer’s Disease (EOAD) who screened negative for disease-causing genetic mutations.
The PBMC clustering generated 17 primary clusters containing all the expected PBMC types. When comparing the relative cluster abundance in cases vs. controls, Cluster 15 stood out for its robust expansion. It was later revealed that Cluster 15 represented a subtype of CD4 T cells. Noticeably, most of the differences in cluster expansion in EOAD patients were primarily driven by females. This population of CD4 T cells exhibited high levels of genes involved in interferon (IFN) signaling leading to the classification of this cluster as ‘Interferon Signaling-Associated Gene high (ISAGhi) T cells’. These cells exhibit a similar gene expression profile to T-cell subtypes expanded in the cerebrospinal fluid of patients with viral encephalitis (Heming, 2021). Therefore, it is hypothesized that these ISAGhi T cells have antiviral characteristics and contribute to T-Cell activation. In addition, the researchers observed upregulation of IFN-signaling genes across additional lymphoid and myeloid PBMC types in EOAD. ISAGhi T cell abundance was found to be consistent across scRNA-seq batches and unaffected by genetic variants like APOE ε4. No relationship was found between age and ISAGhi T cell abundance.
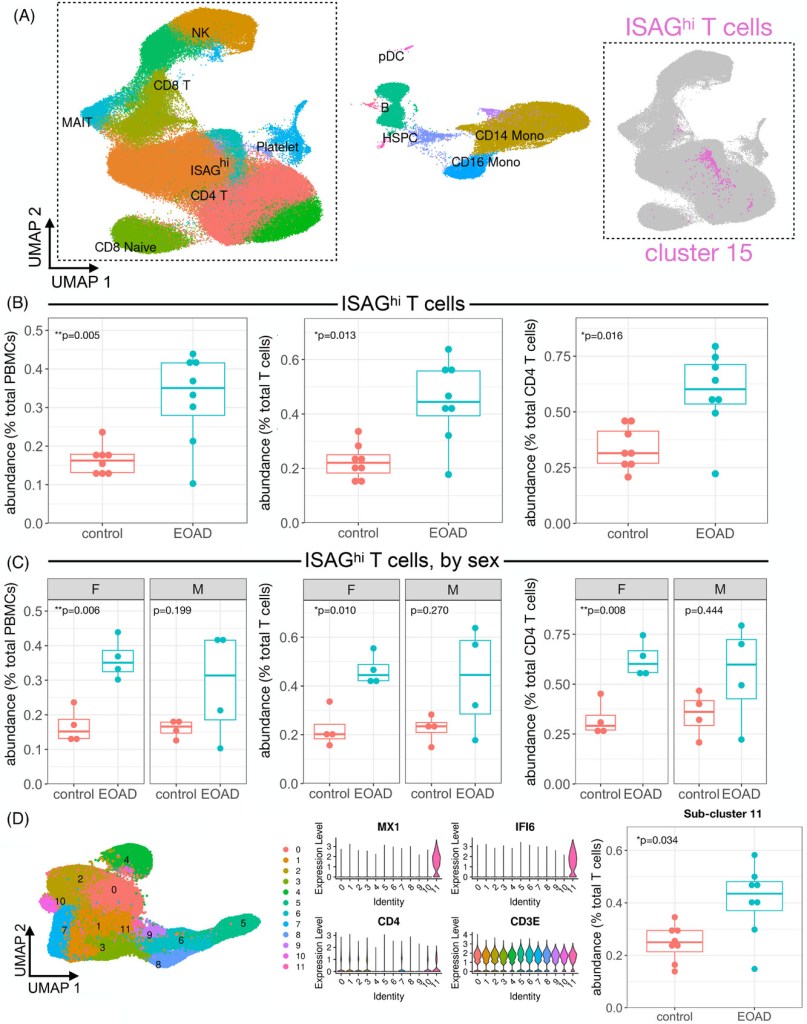
In a later stage, the researchers compared the EOAD and familial tauopathy (carriers of MAPT pathogenic variants) scRNA seq of peripheral non-classical monocyte datasets, the researchers found no significant expression of ISAGhi T Cells in familial tauopathy, suggesting different immune responses between these two groups. This difference in immune response in EOAD and familial tauopathy was corroborated by observations of natural killer (NK) cell clusters. There was a significant proliferation in EOAD (due to heightened peripheral interferon signaling), especially in females, but no significant changes in proliferating NK cells in the familial tauopathy dataset.
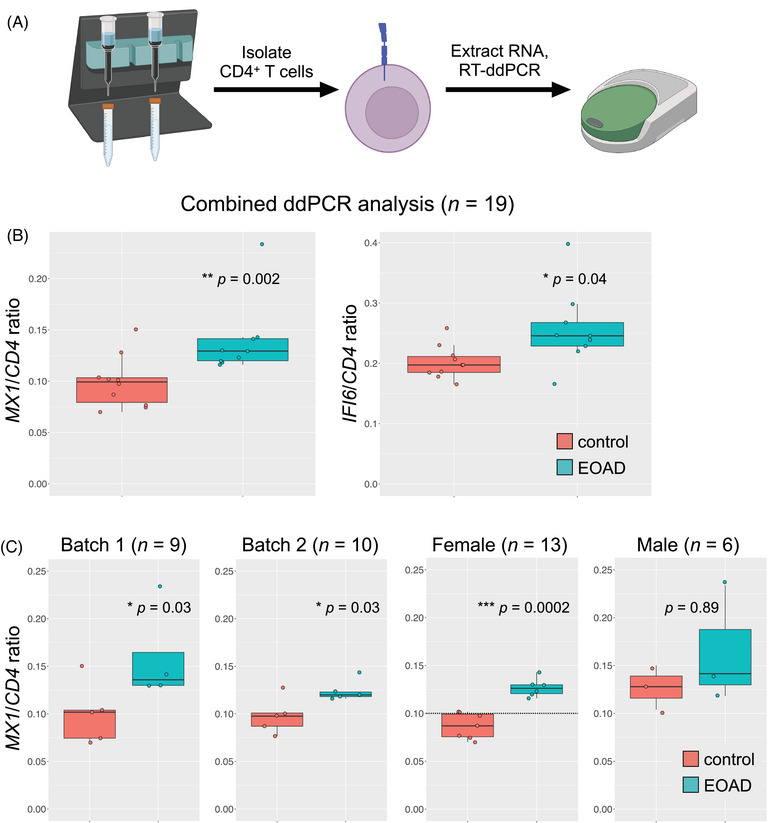
To validate their initial findings, researchers assessed ISAGhi marker genes in a second cohort of EOAD and healthy participants through droplet digital PCR. They found increased expression of the MX1 and IFI6 antiviral genes. Most notably, increased MX1 expression was consistent with previous findings and was driven by females in this cohort as well. Researchers did not find these ISAGhi T cell expansion in late onset mild cognitive impairment (MCI) or AD patients, suggesting that this may be unique to EOAD even though upregulation of the same interferon signaling pathway is conserved in late onset MCl/AD.
Ultimately, the study’s findings suggest a novel role for antiviral T cells in EOAD. They found evidence for a unique peripheral immune signature in EOAD, and the results largely suggest the importance of heightened IFN signaling in PBMCs and its possible relation with AD neurodegeneration. Despite the small sample size limitation, this work brings attention to biological mechanisms underlying sex-specific neurodegeneration vulnerability and relevance of pro-inflammatory cascades.
Sirkis, D. W., Warly Solsberg, C., Johnson, T. P., Bonham, L. W., Oddi, A. P., Geier, E. G., Miller, B. L., Rabinovici, G. D., & Yokoyama, J. S. (2024). Expansion of highly interferon‐responsive T cells in early‐onset alzheimer’s disease. Alzheimer’s & Dementia, 20(7), 5062–5070. https://doi.org/10.1002/alz.13892

